orbital diagram arsenic
Located in the IV period. 1 Using aufbau principle 2 Using periodic table 3 From its bohr model 4 From its orbital.

Arsenic As
According to the Pauli Exclusion Principle two electrons in an orbital will not spin the same.

. Arsenic comes from apple seeds where it is stored as one of your bodys necessary nutrients. Atomic orbital diagram for arsenic Arsenic ion As 3- electron configuration The ground state electron configuration of arsenic is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 3. In larger amounts however arsenic is dangerous and even deadly.
What is the orbital. This electron configuration shows that the last shell of arsenic has five electrons. As Arsenic is an element with position number 33 in the periodic table.
Arsenic has 33 electrons including 3 in its outermost. We can write the electron configuration of arsenic using four different methods. What is the orbital diagram of arsenic.
To write the orbital diagram for the Arsenic atom As first we need to write the electron configuration for just As. The orbital diagram of arsenic shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons the 2p subshell has 6 electrons the 3s subshell has 2 electrons the. Therefore the valence electrons of arsenic are five.
Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. Electronic configurations of elements. To do that we need to find the number of electrons for the As.
The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. When there are two electrons in an orbital the electrons are called an electron pair. Arsenic ground state configuration electron diagram orbital atom electrons valence definition example number shell summary Homework Study Helpers.
Orbital diagrams Orbital box diagrams of all elements are mentioned in the. Answer Bank Energy Identify the charges of the two monoatomic ions an anion and a cation. 1 By referring to a periodic table we can see that arsenic As has an atomic number of 33 which tells us it has 33 protons and 33 electrons in its neutral state.
Arsenic has 33 electrons including 3 in its outermost shell. The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. Construct the orbital diagram for arsenic to show the full ground-state electron configuration.
Sulfur K Edge X Ray Absorption Spectroscopy And Time Dependent Density Functional Theory Of Arsenic Dithiocarbamates Dalton Transactions Rsc Publishing Doi 10 1039 C4dt00078a

File Electron Configuration Bromine Svg Wikimedia Commons

Electron Configuration Atom Electron Affinity Arsenic Periodic Table Compare Angle White Text Png Pngwing

Ionization Energies Mike Jones Pisgah High School Canton Nc Ppt Video Online Download

Electron Configurations Of Atoms

Webelements Periodic Table Arsenic Properties Of Free Atoms

Show The Full Ground State Electron Configuration Of Arsenic By Building Its Orbital Diagram What Are The Charges Of The Monatomic Ions Most Likely To Be Formed Select Two Charges An Anion
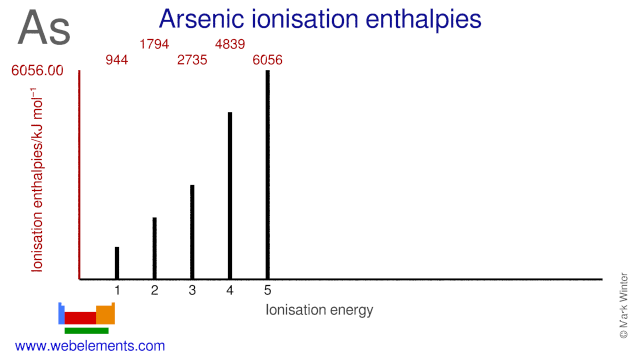
Webelements Periodic Table Arsenic Properties Of Free Atoms

Draw The Orbital Diagram For Arsenic Homework Study Com
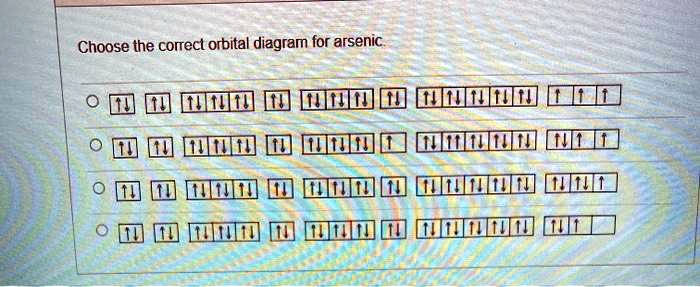
Solved Choose The Correct Orbital Diagram For Arsenic

Orbital Filling Diagrams Youtube
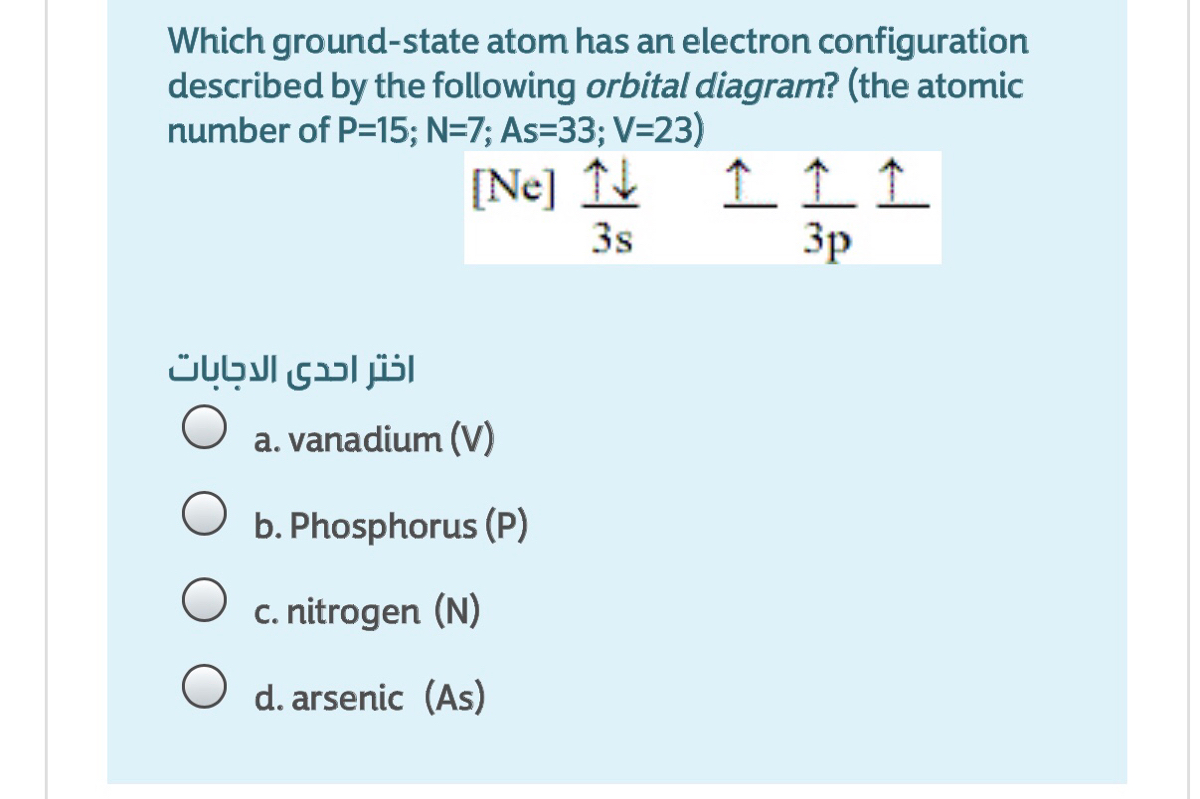
Answered Which Ground State Atom Has An Electron Bartleby

Arrange The Electrons In The Sulfur Atom In The Orbital Diagram Use The Periodic Table To Determine Brainly Com

Write Electron Configuration For Arsenic Youtube
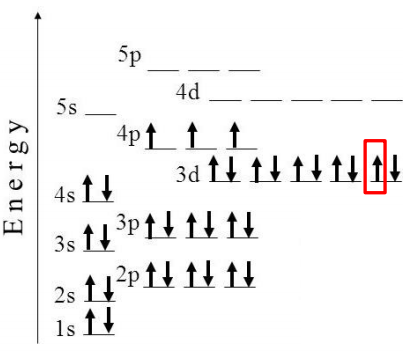
Solved Below Is The Orbital Diagram For Arsenic 33as Chegg Com

Orbital Diagrams Electron Configuration Shorthand Electron Configuration The Atom Ii Periodic Table Quizlet Live Diagram Quizlet
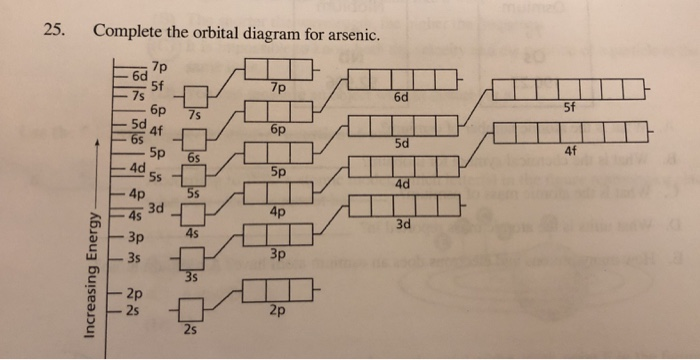
Solved 25 Complete The Orbital Diagram For Arsenic 7p 5f Chegg Com